New modelling platform benefits from ADDoPT input
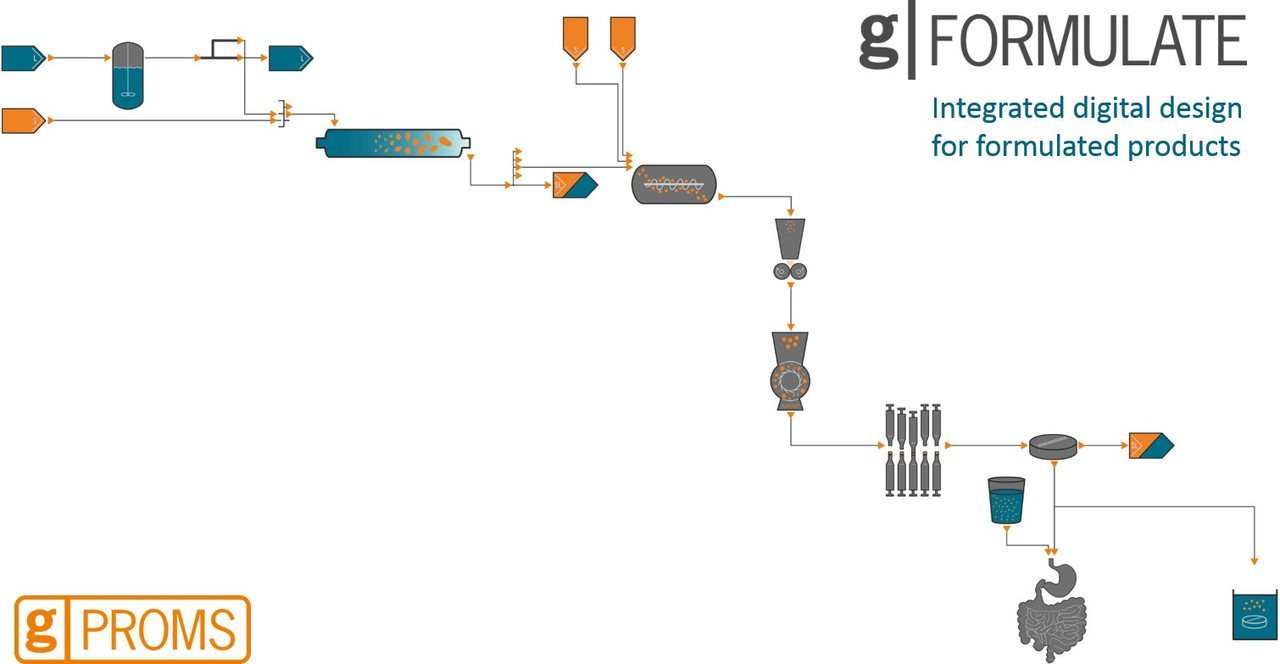
PSE's gPROMS FormulatedProducts® promises integrated mechanistic modelling from formulation to product performance
ADDoPT lead partner Process Systems Enterprise (PSE), has just launched its new gPROMS FormulatedProducts modelling platform for the integrated digital design of robust formulated products and their manufacturing processes. Developed in close collaboration with leading pharmaceutical, agrochemical, consumer products and food organisations, gPROMS FormulatedProducts deploys a mechanistic modelling approach to enable scientists and engineers to screen formulations with complex phase structures for quality attributes, determine whether they can be manufactured robustly, and comprehensively explore the design space for the whole formulation and manufacturing chain.
In particular, the platform helps pharmaceutical companies optimise the formulation and manufacture of drug substances and drug products using mechanistic models of material, unit operations and product performance. This enables them to screen new formulations faster against quality attributes including in vivo performance and manufacturability. According to PSE, it also accelerates tech transfer whilst reducing risk and improving R&D efficiency, and facilitates better capture and transfer of corporate knowledge across the organisation. Built on PSE’s gPROMS® modelling platform, gPROMS FormulatedProducts includes extensive libraries of mechanistic models for operations such as reaction, crystallisation, wet and dry milling, spray drying, wet and dry granulation, blending and tableting. It also provides databases and calculation methods for physical properties, material properties, equipment and physiology.
The development of gPROMS FormulatedProducts has benefited directly from work carried out in WP1 of ADDoPT to develop a systems framework for pharmaceutical manufacture, linking all the tasks in the pharmaceutical workflow from a molecule to a drug dosage form, and the signs are promising for further progress. Developments were and continue to be informed by cross linkages to other parts of the project, such as WP4 where fundamental and industrial research into all the main stages of late stage primary and secondary pharmaceutical manufacture is providing insight into the links between raw materials, manufacturing processes and the needs of the patient, and WP6 where PSE are working with other partners, including Britest and Perceptive Engineering Limited, to create a new generation of model predictive controllers and associated monitoring technology, making use of hybrid model structures to allow robust control/monitoring of continuous and batch pharmaceutical production.
Sean Bermingham, head of PSE Formulated Products said, “We have benefited enormously from the feedback we received from PSE’s Formulated Products Advisory Board as well as the input from the Systems-based Pharmaceutics Alliance and our industrial and academic partners in major R&D collaborations such as ADDoPT, C-SOPS, CMAC, D3P and REMEDIES. This has resulted in a tool based on state-of-the-art science that can address practical challenges”.
More on gPROMS FormulatedProducts at PSE's website.