ADDoPT engages with Pharma R&D Community at APS Digital Design Symposium
Over 80 researchers and industrialists involved in pharmaceutical development and manufacturing gathered for the Academy of Pharmaceutical Sciences Symposium on the Digital Design of Drug Products on Friday 1st July 2016.
 Hosted by ADDoPT partners from the University of Leeds and chaired by AstraZeneca materials scientist, APS Treasurer, and ADDoPT lead researcher Dr. Richard Storey, the symposium was the culmination of a week of activity built around the joint CGOM12 / BACG 2016 Crystal Growth conferences. These events provided a dynamic forum for broad scientific and technological exchange around the fundamentals of crystal growth, different types of crystals and their crystallisation environment, crystallisation engineering and crystallisation characterisation techniques. A primary aim of the
Digital Design Symposium was to provide an update on the current state of modelling and prediction tools in academia and what are the key challenges from industry providing the pull for their use.. The ADDoPT project is perfectly placed to help link these audiences together and produce better tools for processes.
Hosted by ADDoPT partners from the University of Leeds and chaired by AstraZeneca materials scientist, APS Treasurer, and ADDoPT lead researcher Dr. Richard Storey, the symposium was the culmination of a week of activity built around the joint CGOM12 / BACG 2016 Crystal Growth conferences. These events provided a dynamic forum for broad scientific and technological exchange around the fundamentals of crystal growth, different types of crystals and their crystallisation environment, crystallisation engineering and crystallisation characterisation techniques. A primary aim of the
Digital Design Symposium was to provide an update on the current state of modelling and prediction tools in academia and what are the key challenges from industry providing the pull for their use.. The ADDoPT project is perfectly placed to help link these audiences together and produce better tools for processes.
Against this backdrop, the .symposium provided a vivid picture of how new Digital Design concepts in materials engineering and state of the art materials and process modelling are being used to harness data from across the pharmaceutical arena, in order to deliver on the aspiration of Quality by Design. Prospective benefits include speeding up the delivery of new products, improved product robustness, and optimised material properties (rather than accommodation of the sub-optimal).
A series of distinguished international speakers from both academia and industry developed the day’s theme, starting from the academic vision of ‘The Materials Science Tetrahedron’ described by keynote speaker Professor Calvin Sun, of the University of Minnesota. Professor Sun’s ideas, summarised in an influential 2009 paper in the Journal of Pharmaceutical Sciences, argue that a systematic implementation of design principles built on recognition and understanding of the interdependencies of the structure, properties, performance, and processing of drugs is needed to drive the transformation of pharmaceutical product development from an art to a science.
New Modelling Capabilities
Speakers from the UK academic community illustrated how new modelling capabilities, built on an understanding of the underlying mechanisms of key physical processes at a range of size scales, are already bringing the vision closer to reality in areas including particle breakage, wet granulation and compaction. Pointing out that the fracture of most pharmaceutical APIs is strain-rate sensitive due to their visco-elastic nature, Professor Mojtaba Ghadiri of the University of Leeds described how effective modelling of particle impact velocity in a commercial Malvern 2000 Scirocco dry dispersion unit had enabled its adaption to elucidate fracture / particle size relationships. Professor Ghadiri’s team will be pursuing further velocity modelling of industry relevant milling environments within one of ADDoPT’s Work Package 4 sub-streams
Professor Jim Litster, jointly Professor of Chemical and Pharmaceutical Engineering at the University of Sheffield and Industrial and Physical Pharmacy at Purdue University, described progress along a knowledge-led pathway from conventional experimental design to fully predictive modelling in the complex area of wet granulation. Improved understanding of granulation rate processes through the use of regime mapping based on dimensionless groups is allowing more scaleable processes and more effective troubleshooting. In Professor Litster’s view, the transition from the current state of understanding of control mechanisms to fully predictive modelling is underway, and will be based on population balance approaches. Such approaches are already being implemented in commercially available modelling software such as PSE’s gPROMS / gSOLIDS.
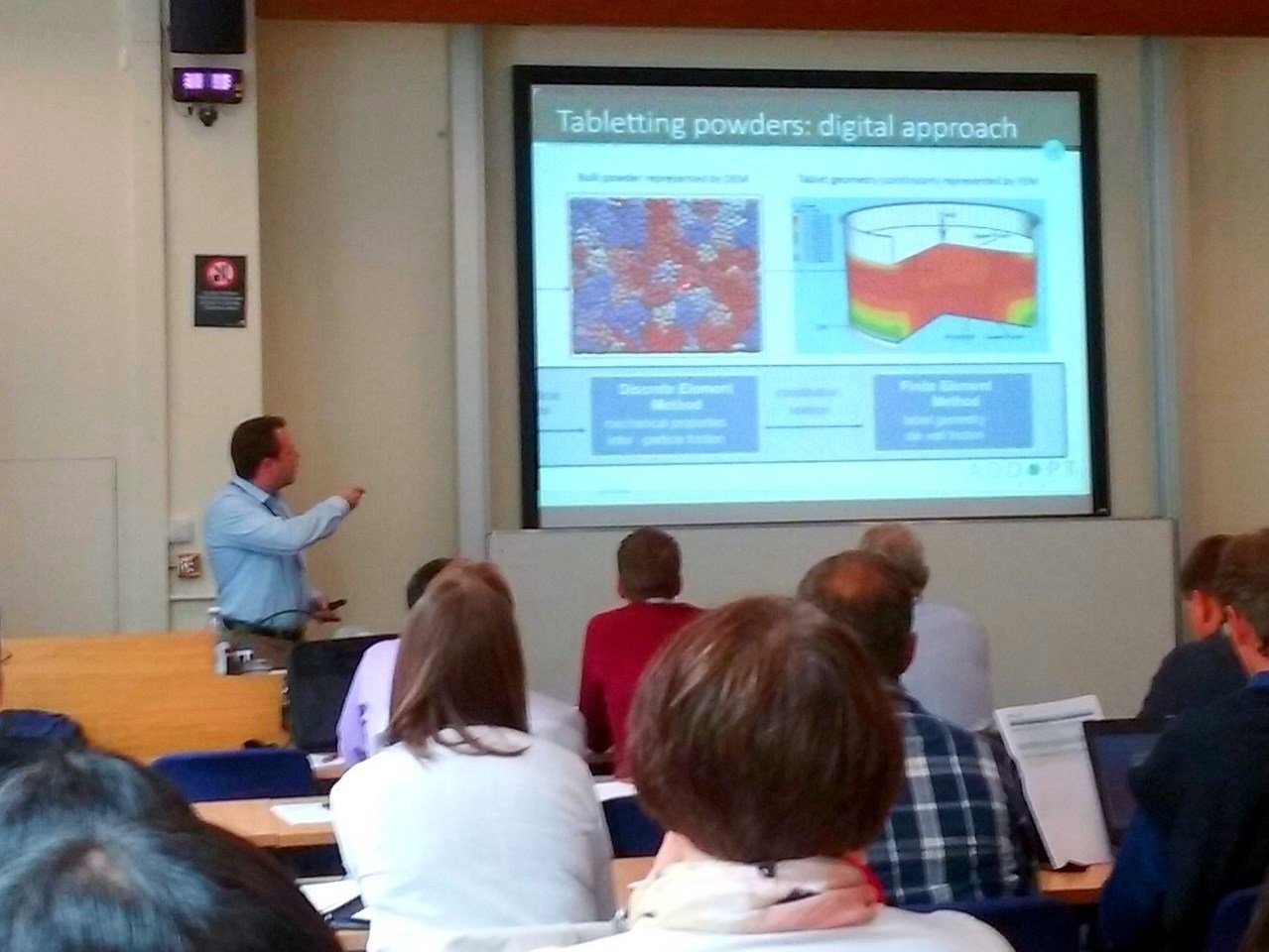 Dr. James Elliott, leader of Cambridge University’s research team in ADDoPT, presented recent results built upon more computationally efficient methods for predictive modelling of compression incorporating adhesion. These demonstrated successful simulations of triclinic (stable) and monoclinic crystal forms of acetazolamide which were consistent with the observed tablettability of the two polymorphs. Progress with this sort of multiscale workflow approach (from the molecular /quantum scale through the crystal to the bulk tablet) led Dr. Elliott to believe that predicting tablettability from molecular structure is now a realistic goal. Necessary further work, which ADDoPT will enable, includes extending the approach to a broader range of compounds, taking account of surface energy anisotropy effects, and the effect of crystal defects on mechanical properties such as yield stress, an area in which ADDoPT collaboration with specialists at Leeds adds value to the research.
Dr. James Elliott, leader of Cambridge University’s research team in ADDoPT, presented recent results built upon more computationally efficient methods for predictive modelling of compression incorporating adhesion. These demonstrated successful simulations of triclinic (stable) and monoclinic crystal forms of acetazolamide which were consistent with the observed tablettability of the two polymorphs. Progress with this sort of multiscale workflow approach (from the molecular /quantum scale through the crystal to the bulk tablet) led Dr. Elliott to believe that predicting tablettability from molecular structure is now a realistic goal. Necessary further work, which ADDoPT will enable, includes extending the approach to a broader range of compounds, taking account of surface energy anisotropy effects, and the effect of crystal defects on mechanical properties such as yield stress, an area in which ADDoPT collaboration with specialists at Leeds adds value to the research.
The Industrial Perspective
The afternoon session turned its attention to the industrial context, both through the viewpoint and needs of the pharma sector towards Digital Design, and to the latest developments in state of the art modelling software. Dr. Kendal Pitt, Senior Technical Director in the Centre of Excellence for Solid Dosage Forms at GlaxoSmithKline, led off the industrial perspective by outlining the influential “Proposal for a Drug Product Manufacturing Classification System (MCS) for Oral Solid Dosage Forms” on which he co-authored in 2015. (Pharm Dev Technol 2015, 20(1), 12-21). The MCS is intended as a tool for pharmaceutical scientists to rank the feasibility of different processing routes for the manufacture of oral solid dosage forms, based on selected properties of the API and the needs of the formulation. Interest in this concept and approach has been such that the paper was the number one download request from Pharmaceutical Development and Technology in 2015.

Afternoon keynote speaker Dr. Amrit Paudel, from the Research Centre for Pharmaceutical Engineering, Graz, explained how predictive methods based on understanding key relationships in areas such as drug-excipient interactions, small molecule degradants, crystal habit, and packaging for final product, are enabling chemical stability studies for solid state materials to move on from the limited, and time-consuming conventional practice of forced degradation studies in solution.
Particle Perfection or Practical Perfection?
Symposium Chair Richard Storey kicked off a lively discussion with a presentation reflecting on steps “Towards the Perfect Particle”, which summarised thoughts from the four pharmaceutical manufacturing companies represented within ADDoPT on topics ranging from what are the important particle design attributes to consider, through to what factors and influences drive thinking and behaviour in industry either towards or away from successfully taking up the new Digital Design paradigm. In concluding that perfection was neither possible nor in fact desirable, Dr. Storey summarised that more thoroughly understood physical property relationships were clearly important (but variability was inevitable); concepts like constructing a “particle passport” would allow optimisation of surface properties to enhance dissolution and physical properties; and the MCS could provide vital consistency in guidance for numerous companies. Further contributions from the floor developed thoughts around the need for, and organisational and cultural challenges associated with, data management if better models are to be realised.
Professor Costas Pantelides, Managing Director of ADDoPT lead partner Process Systems Enterprise, brought the day to a conclusion with a presentation laying out both the ADDoPT vision for system-based pharmaceutics as a means of establishing an efficient, knowledge-based, Quality-by-Design oriented supply chain for the UK pharmaceuticals sector, and PSE’s own contribution to the practical implementation of system-level modelling capabilities through product developments such as the forthcoming gPROMS FormulatedProducts platform for the design of formulated products and their manufacturing processes.
Overall, the Digital Design Symposium provided an excellent platform for reviewing the Digital Design landscape relating to pharmaceutical product development and manufacture and catalysed exchange of views, information and discussion between a broad range of interested parties. This engagement and exchange needs to continue, and it is hoped to hold a similar event next year where further updates on progress can be anticipated.
Post script: Selected presentations from the APS Digital Design Symposium may be found amongst post-event resources for the CGOM/BACG conference week here.
8th July 2016